Observational Cohort
The observational cohort was intended to observe natural brain changes and cognitive performance over time.
After confirmation of eligibility (after telephone screening, eligibility and enrolment visits) our research participants underwent their ‘baseline’ visit which was repeated annually afterward. See section ‘measuring disease progression’ for the description of the evaluations.
Main Inclusion criteria :
- 55 years old or more
- First-degree relative affected by Alzheimer’s Disease
- Cognitively normal
- Stable general health
See below for detailed inclusion/exclusion criteria
Visit schedule:
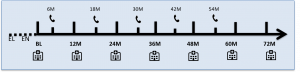
EL: eligibility visit; EN: enrolment visit; BL: Baseline visit; M: months;

Recruitment:
Started November 2011 – Ended May 2017
- We enrolled 425 participants in the PREVENT-AD program and now continue to follow 330 participants annually.
Detailed inclusion & exclusion criteria
| Inclusion criteria |
- Parental or multiple-sibling history of Alzheimer-like dementia
- Age 60 years or older (persons aged 55-59 years and < 15 years younger than their affected index relative were also eligible.)
- Minimum of 6 years of formal education
- Study partner available to provide information on cognitive status
- Sufficient fluency in spoken and written English and/or French
- Ability and intention to participate in regular visits
- Provision of informed consent
- Agreement for periodic donation of blood and urine samples
- Agreement to participate in periodic multi-modal assessments via MRI and neurosensory testing, and (optional and later on obligatory) LPs for CSF collection,
- Agreement to limit use of medicines as required by investigational protocols
|
| Exclusion criteria |
- Known or identified cognitive disorder
- Use of acetyl-cholinesterase inhibitors including tacrine, donepezil, rivastigmine, galantamine
- Use of memantine or other approved prescription cognitive enhancer
- Use of vitamin E at greater than 600 i.u. / day or aspirin at >325 mg / day
- Use of opiates (oxycodone, hydrocodone, tramadol, meperidine, hydromorphone)
- Use of NSAIDs or of systemic or inhalation corticosteroids
- Clinically significant hypertension (OK if controlled medically), anemia, significant liver or kidney disease
- Concurrent use of warfarin, ticlopidine, clopidrogel, or similar anti-coagulant
- Current plasma Creatinine >1.5 mg/dl (132 mmol/l)
- Current alcohol, barbiturate or benzodiazepine abuse/dependence
|
CSF: cerebrospinal fluid; MRI: magnetic resonance imaging; NSAID: non-steroidal anti-inflammatory drug; LP: lumbar puncture

